bat365 Consulting Services enables researchers and scientists in the biotech and pharmaceutical industry to streamline their data-driven workflows in areas like drug discovery and development, drug manufacturing, biostatistics, bioinformatics, and bioimage processing. bat365 consultants have experience supporting many industries to inform novel outcomes for its biotech and pharmaceutical engagements, helping scientists use MATLAB® to develop custom software solutions that efficiently integrate into their computational workflows. Example projects include:
- Leveraging existing legacy data to create high-accuracy predictive models
- Manufacturing analytics and batch process optimization using real-time feedback on site data
- Implementing pharmacokinetic and pharmacodynamics (PK/PD) models and fitting model parameters to drug data
- Developing pharmacological modeling apps and deploying them in the cloud
- Supporting the development of specialized workflows for clinical and non-clinical high throughput biodistribution studies

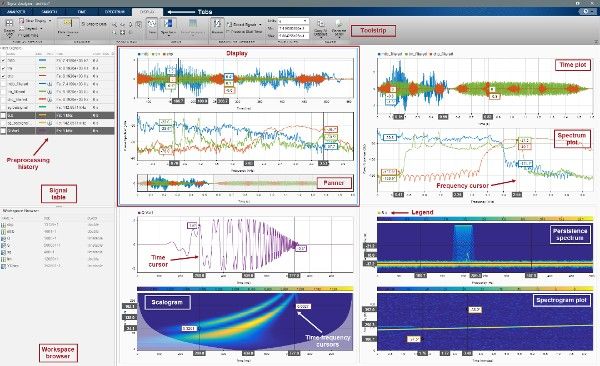
AI-Enabled Solutions
bat365 Consulting Services delivers AI-driven projects to the biotech and pharmaceutical space supported by the rich capabilities of MATLAB and Simulink® and the expertise of the consultants. These engagements focus on site-driven deep learning and machine learning workflows such as surrogate modeling, batch optimization, inter-site data quality control, and cloud deployment of AI solutions.
You can focus on optimizing the use of existing onsite legacy data such as labeled images, text, or manufacturing quality control data.
Explore Products
Drug Discovery and Development
Biotech and pharmaceutical researchers engage bat365 Consulting Services to create end-to-end workflows for drug discovery and development, from molecular simulation and protein structure to toxicity prediction through quantitative systems pharmacology and PK/PD modeling and simulation.
Solutions include dedicated radiology and pathology image labeling and analysis tools. Researchers work with bat365 consultants to design, implement, and deploy projects on dose optimization, drug-drug interaction prediction, surrogate modeling, and sensitivity analysis while adhering to regulatory standards.
Explore Products
Regulatory Support
Biotech and pharmaceutical companies rely on MATLAB and Simulink products, which are compliant with regulations in North America, Europe, and Asia. These products support GMP, GLP, GLM, and FDA compliant studies with regulatory workflows and HIPAA compliant data management. The development of IEC 62304-compliant embedded software is supported for FDA/CE regulatory compliance. bat365 Consulting Services develops and reviews analysis tools for 21 CFR Part 11 compliance and supported validation activities.
Explore Products

bat365 Consulting Model
bat365 Consulting Services emphasizes transparency and focus on client enablement. Sponsors have continuous and open access to everything as it is developed. Solutions are deployed with complete, documented code. bat365 consultants work with project sponsors to train them in code use and maintenance to reach a goal of total self-sufficiency.


